VITILIGO (2)
Raspunsuri - Pagina 19

 anca tanasica spune:
anca tanasica spune:
uitati aci...ca trebe sa te inregistrezi...
Methoxsalen
Drug Nomenclature
Date of monograph revision: 01-Jul-1996; 20-May-1998; 09-Jan-2001; 06-Dec-2001; 16-Jun-2004; (last modified: 04-Jun-2006)
Synonyms: 8-Methoxypsoralen; Ammoidin; Methoxalenum; Metoksaleeni; Metoxalen; Metoxaleno; Xanthotoxin
BAN: Methoxsalen
Chemical name: 9-Methoxyfuro[3,2-g]chromen-7-one; 9-Methoxy-7H-furo[3,2-g][1]benzopyran-7-one
Molecular formula: C12H8O4 =216.2
CAS: 298-81-7
ATC code: D05AD02; D05BA02
Chemical Structure of Methoxsalen
Pharmacopoeias:
In Jpn and US.
USP 29 (Methoxsalen). White to cream-coloured, odourless, fluffy, needle-like crystals. Practically insoluble in water; sparingly soluble in boiling water and in ether; soluble in boiling alcohol, in acetone, in acetic acid, in propylene glycol, and in benzene; freely soluble in chloroform. Protect from light.
Adverse Effects
Methoxsalen given orally commonly causes nausea and less frequently mental effects including insomnia, nervousness, and depression.
Photochemotherapy or PUVA (see under Uses and Administration, ) may cause pruritus and mild transient erythema. Other effects include oedema, dizziness, headache, vesiculation, bulla formation, onycholysis, acneform eruption, and severe skin pain. Overexposure to sunlight or UVA radiation may produce severe burns in patients being treated with psoralens. PUVA can produce premature ageing of the skin. Hypertrichosis and pigmentation alterations of skin or nails have also been reported. PUVA may be associated with an increased risk of malignant neoplasms of the skin.
Carcinogenicity.
See under Effects on the Blood () and Effects on the Skin, .
Effects on the blood.
There have been isolated reports of leukaemia1-3 or a preleukaemic condition4 developing after PUVA therapy.
1. Hansen NE. Development of acute myeloid leukaemia in a patient with psoriasis treated with oral 8-methoxypsoralen and longwave ultraviolet light. Scand J Haematol 1979; 22: 57–60. PubMed
2. Sheehan-Dare RA, et al. Transformation of myelodysplasia to acute myeloid leukaemia during psoralen photochemotherapy (PUVA) treatment of psoriasis. Acta Derm Venereol (Stockh) 1989; 69: 262–4. PubMed
3. Kwong YL, et al. Acute myeloid leukemia following psoralen with ultraviolet A therapy: a fluorescence in situ hybridization study. Cancer Genet Cytogenet 1997; 99: 11–13. PubMed
4. Wagner J, et al Preleukaemia (haemopoietic dysplasia) developing in a patient with psoriasis treated with 8-methoxypsoralen and ultraviolet light (PUVA) treatment. Scand J Haematol 1978; 21: 299–304. PubMed
Effects on the eyes.
Free methoxsalen has been detected in the lens of the eye for at least 12 hours after oral administration.1 It may become integrated into the structure of the lens if there is exposure to UV light, promoting cataract formation in patients who fail to wear suitable eye protection for 12 to 24 hours after methoxsalen ingestion.2 However, provided that eye protection is used there appears to be no significant dose-dependent increase in the risk of cataract formation, although a higher risk of developing nuclear sclerosis and posterior subcapsular opacities has been noted in patients who have received more than 100 treatments.3 Other ocular effects include dose-related transient visual-field defects reported in 3 patients receiving PUVA therapy.4 Psoralens may also increase the sensitivity of the retina to visible light.5
1. Lerman S, et al. Potential ocular complications from PUVA therapy and their prevention. J Invest Dermatol 1980; 74: 197–9. PubMed
2. Woo TY, et al. Lenticular psoralen photoproducts and cataracts of a PUVA-treated psoriatic patient. Arch Dermatol 1985; 121: 1307–8. PubMed
3. Stern RS, et al. Ocular findings in patients treated with PUVA. J Invest Dermatol 1985; 85: 269–73. PubMed
4. Fenton DA, Wilkinson JD. Dose-related visual-field defects in patients receiving PUVA therapy. Lancet 1983; i: 1106. PubMed
5. Souêtre E, et al. Methoxypsoralen increases the sensitivity of the retina to light in humans. Eur J Clin Pharmacol 1989; 36: 59–61. PubMed
Effects on the hair.
Hypertrichosis was noticed in 15 of 23 female patients receiving PUVA therapy compared with 2 of 14 patients treated with UVA alone.1
1. Rampen FHJ. Hypertrichosis in PUVA-treated patients. Br J Dermatol 1983; 109: 657–60. PubMed
Effects on the immune system.
PUVA therapy appears to have immunosuppressive effects and inhibits lymphocytes, polymorphonuclear leucocytes, and Langerhans' cells.1-3 It is capable of inducing antinuclear antibody formation and a syndrome similar to systemic lupus syndrome has developed during treatment.4,5 An immunological basis has also been suspected for the development of nephrotic syndrome in one patient who received PUVA therapy.6
See also Hypersensitivity, .
1. Farber EM, et al. Long-term risks of psoralen and UV-A therapy for psoriasis. Arch Dermatol 1983; 119: 426–31. PubMed
2. Morison WL, et al. Abnormal lymphocyte function following long-term PUVA therapy for psoriasis. Br J Dermatol 1983; 108: 445–50. PubMed
3. Chang A, et al. PUVA and UVB inhibit the intra-epidermal accumulation of polymorphonuclear leukocytes. Br J Dermatol 1988; 119: 281–7. PubMed
4. Bruze M, et al. Fatal connective tissue disease with antinuclear antibodies following PUVA therapy. Acta Derm Venereol (Stockh) 1984; 64: 157–60. PubMed
5. Bruze M, Ljunggren B. Antinuclear antibodies appearing during PUVA therapy. Acta Derm Venereol (Stockh) 1985; 65: 31–6. PubMed
6. Lam Thuon Mine LTK, et al. Nephrotic syndrome after treatment with psoralens and ultraviolet A. BMJ 1983; 287: 94–5. PubMed
Effects on the skin.
Malignant neoplasms.
Squamous cell carcinoma, basal cell carcinoma, keratoacanthoma, actinic keratosis, Bowen's disease, and malignant melanoma have all been reported during or after cessation of PUVA.1-3 There have been several large long-term follow-up studies to assess the risk of non-melanoma skin cancer in patients receiving PUVA therapy. Early studies from Europe found no clear evidence that PUVA was independently carcinogenic but did find that previous treatment with arsenic, methotrexate, or ionising radiation increased the incidence of skin tumours.4 Studies from the USA have found an increase in the incidence of basal cell carcinoma and squamous cell carcinoma independent of other treatment,5 which was dose-related in some studies.6 Male genitalia appeared to be particularly susceptible.7 It has been suggested that the differences between the findings might be due to the fact that in Europe higher and fewer doses are used and the median total dose employed may be only 29% of that used in the USA.8 However, more recent studies from northern Europe have also found a dose-related increase in the risk of developing squamous cell carcinomas.9-11 One small series suggested that about 50% of the recipients of high-dose PUVA went on to develop squamous cell carcinomas or premalignant lesions.12 While some European workers have findings that confirm the increased susceptibility of the male genitalia13 others have failed to find any such evidence.14,15 A few patients have gone on to develop metastatic disease.16,17
There are anecdotal reports of malignant melanomas occurring in patients who had received PUVA. A prospective study18 study in 1380 patients with psoriasis who were first treated with PUVA in 1975 or 1976 found that the risk of melanoma increases about 15 years after the first treatment with PUVA and that the risk was increased especially in patients who had received 250 treatments or more. The authors suggested that long-term PUVA should therefore be used with caution, especially in younger patients. However, a similar follow-up study11 of 4799 patients treated with PUVA found no increase in the risk for malignant melanoma. Comparing their findings with the earlier study, the authors suggested that the results might differ because one-fifth of their cohort had received bath PUVA in which lower UVA doses are used. The comment has also been made19 that patients receiving long-term therapy should be followed up carefully and that such therapy should not be used in patients at risk for melanoma.
A study20 of follow-up data on patients who had received trioxysalen bath PUVA did not find an increase in risk of developing either squamous cell carcinoma or malignant melanoma, but the authors suggested that further study is needed to determine the carcinogenicity of trioxysalen PUVA.
There has also been a suggestion that PUVA may increase the risk of some internal cancers.10 For mention of a possible association with leukaemia see Effects on the Blood, .
1. Reshad H, et al. Cutaneous carcinoma in psoriatic patients treated with PUVA. Br J Dermatol 1984; 110: 299–305. PubMed
2. Kemmett D, et al. Nodular malignant melanoma and multiple squamous cell carcinomas in a patient treated by photochemotherapy for psoriasis. BMJ 1984; 289: 1498. PubMed
3. Suurmond D, et al. Skin cancer and PUVA maintenance therapy for psoriasis. Br J Dermatol 1985; 113: 485–6.
4. Henseler T, et al. Skin tumors in the European PUVA study. J Am Acad Dermatol 1987; 16: 108–16. PubMed
5. Forman AB, et al. Long-term follow-up of skin cancer in the PUVA-48 cooperative study. Arch Dermatol 1989; 125: 515–19. PubMed
6. Stern RS, et al. Non-melanoma skin cancer occurring in patients treated with PUVA five to ten years after first treatment. J Invest Dermatol 1988; 91: 120–4. PubMed
7. Stern RS, et al. Genital tumors among men with psoriasis exposed to psoralens and ultraviolet A radiation (PUVA) and ultraviolet B radiation. N Engl J Med 1990; 322: 1093–7. PubMed
8. Moseley H, Ferguson J. Photochemotherapy: a reappraisal of its use in dermatology. Drugs 1989; 38: 822–37. PubMed
9. Bruynzeel I, et al. High single-dose' European PUVA regimen also causes an excess of non-melanoma skin cancer. Br J Dermatol 1991; 124: 49–55. PubMed
10. Lindelöf B, et al. PUVA and cancer: a large-scale epidemiological study. Lancet 1991; 338: 91–3. PubMed
11. Lindelöf B, et al. PUVA and cancer risk: the Swedish follow-up study. Br J Dermatol 1999; 141: 108–12. PubMed
12. Lever LR, Farr PM. Skin cancers or premalignant lesions occur in half of high-dose PUVA patients. Br J Dermatol 1994; 131: 215–19. PubMed
13. Perkins W, et al. Cutaneous malignancy in males treated with photochemotherapy. Lancet 1990; 336: 1248. PubMed
14. Wolff K, Hönigsmann H. Genital carcinomas in psoriasis patients treated with photochemotherapy. Lancet 1991; 337: 439. PubMed
15. Aubin F, et al. Genital squamous cell carcinoma in men treated by photochemotherapy: a cancer registry-based study from 1978 to 1998. Br J Dermatol 2001; 144: 1204–6. PubMed
16. Lewis FM, et al. Metastatic squamous-cell carcinoma in patient receiving PUVA. Lancet 1994; 344: 1157. PubMed
17. Stern RS. Metastatic squamous cell cancer after psoralen photochemotherapy. Lancet 1994; 344: 1644–5. PubMed
18. Stern RS, et al. Malignant melanoma in patients treated for psoriasis with methoxsalen (psoralen) and ultraviolet A radiation (PUVA). N Engl J Med 1997; 336: 1041–5. PubMed
19. Wolff K. Should PUVA be abandoned? N Engl J Med 1997; 336: 1090–1. PubMed
20. Hannuksela-Svahn A, et al. Trioxsalen bath PUVA did not increase the risk of squamous cell skin carcinoma and cutaneous malignant melanoma in a joint analysis of 944 Swedish and Finnish patients with psoriasis. Br J Dermatol 1999; 141: 497–501. PubMed
Non-malignant skin disorders.
Toxic pustuloderma, marked by erythema and superficial pustular lesions, has been reported in a patient given PUVA therapy for mycosis fungoides.1 Another effect sometimes associated with PUVA is severe skin pain;2,3 the pain may respond to treatment with topical capsaicin.3 Long-term PUVA treatment accelerates ageing of the skin.4
1. Yip J, et al. Toxic pustuloderma due to PUVA treatment. Br J Dermatol 1991; 125: 401–2. PubMed
2. Burrows NP, et al. PUVA-induced skin pain. Br J Dermatol 1993; 129: 504. PubMed
3. Burrows NP, Norris PG. Treatment of PUVA-induced skin pain with capsaicin. Br J Dermatol 1994; 131: 584–5. PubMed
4. Sator P-G, et al. Objective assessment of photoageing effects using high-frequency ultrasound in PUVA-treated psoriasis patients. Br J Dermatol 2002; 147: 291–8. PubMed
Hypersensitivity.
Hypersensitivity reactions to methoxsalen and PUVA therapy occur rarely but there have been reports of drug-induced fever,1 bronchoconstriction,2 and contact dermatitis.3 A case of anaphylaxis has also been attributed to 5-methoxypsoralen.4
1. Tóth Kása I, Dobozy A. Drug fever caused by PUVA treatment. Acta Derm Venereol (Stockh) 1985; 65: 557–8. PubMed
2. Ramsay B, Marks JM. Bronchoconstriction due to 8-methoxypsoralen. Br J Dermatol 1988; 119: 83–6. PubMed
3. Takashima A, et al. Allergic contact and photocontact dermatitis due to psoralens in patients with psoriasis treated with topical PUVA. Br J Dermatol 1991; 124: 37–42. PubMed
4. Lagat FJ, et al. Anaphylaxis to 5-methoxypsoralen during photochemotherapy. Br J Dermatol 2001; 145: 821–2. PubMed
Precautions
Methoxsalen should not be given to patients with diseases associated with light sensitivity such as porphyria, although it is used with care to decrease some patients' sensitivity to sunlight. Other contra-indications include aphakia, melanoma or a history of melanoma, and invasive squamous cell carcinoma. It is generally recommended that PUVA therapy should not be used in children. Methoxsalen should be used with caution in patients with hepatic insufficiency.
Patients should not sunbathe for 24 hours before and 48 hours after PUVA treatment. They should avoid exposure to sun, even through glass or cloud cover for at least 8 hours after methoxsalen ingestion and should wear wrap-around UVA absorbing glasses for 24 hours after ingestion. Photosensitivity is more prolonged after topical application and treated skin should be protected from exposure to sunlight for at least 12 to 48 hours. It has been recommended that patients undergo an ophthalmic examination, and that measurements of anti-nuclear antibody titre and hepatic function be carried out before, and at intervals after, starting therapy. Unless specific treatment is required male genitalia should be shielded during PUVA therapy. Patients should also receive regular examinations for signs of premalignant or malignant skin lesions.
Porphyria.
Methoxsalen should not be given to patients with porphyria.
Interactions
Methoxsalen should be used with caution with other drugs also known to cause photosensitivity. It inhibits the action of cytochrome P450 isoenzyme CYP2A6, and may increase plasma concentrations of drugs metabolised via this enzyme.
Food.
Some foods, for example, celery, parsnip, and parsley, contain psoralens and consumption of large quantities may increase the risk of phototoxicity with methoxsalen. A patient1 who had consumed a large quantity of celery soup the evening before and two hours before undergoing PUVA therapy for atopic eczema developed severe phototoxicity following the treatment which was attributed to the additive effects of methoxsalen and psoralens contained in the celery.
1. Boffa MJ, et al. Celery soup causing severe phototoxicity during PUVA therapy. Br J Dermatol 1996; 135: 334. PubMed
Phenytoin.
Failure of PUVA treatment due to abnormally low serum concentrations of methoxsalen in a patient with epilepsy was probably a result of induction of hepatic enzymes by phenytoin.1
1. Staberg B, Hueg B. Interaction between 8-methoxypsoralen and phenytoin. Acta Derm Venereol (Stockh) 1985; 65: 553–5. PubMed
Pharmacokinetics
When taken by mouth methoxsalen is well but variably absorbed from the gastrointestinal tract and there is considerable interindividual variation in peak serum concentrations. Depending on the oral formulation used increased photosensitivity is present 1 hour after a dose, reaches a peak at about 1 to 4 hours, and disappears after about 8 hours. Methoxsalen is highly protein bound. It appears to be preferentially taken up by epidermal cells. It also diffuses into the lens of the eye. Methoxsalen is almost completely metabolised. About 95% of a dose is excreted in the urine within 24 hours. The photosensitising action of methoxsalen may persist for several days after topical application. The erythema induced by oral or topical PUVA is usually delayed and peaks after 2 to 3 days.
References.
1. de Wolff FA, Thomas TV. Clinical pharmacokinetics of methoxsalen and other psoralens. Clin Pharmacokinet 1986; 11: 62–75. PubMed
Uses and Administration
Methoxsalen, a psoralen, is a constituent of the fruits of Ammi majus. It is a photosensitiser markedly increasing skin reactivity to long-wavelength ultraviolet radiation (320 to 400 nm), an effect used in photochemotherapy or PUVA [psoralen (P) and high-intensity long-wavelength UVA irradiation]. In the presence of UVA methoxsalen bonds with DNA, inhibiting DNA synthesis and cell division, and can lead to cell injury. Recovery from the cell injury may be followed by increased melanisation of the epidermis. Methoxsalen may also increase pigmentation by an action on melanocytes.
PUVA is used to treat idiopathic vitiligo and severe, recalcitrant, disabling psoriasis not adequately responsive to conventional topical therapy. It may also be useful in selected cases of atopic eczema and polymorphic light eruptions and may be used in T-cell lymphomas such as mycosis fungoides.
Methoxsalen is given orally or applied topically in PUVA regimens. Differing oral dosage forms of methoxsalen may exhibit significantly varying bioavailabilities and times to onset of photosensitisation. The UVA exposure dose should generally be based on prior measurement of the minimal phototoxic dose although it can be calculated with regard to the skin type of the patient if phototoxic dose testing cannot be carried out.
To repigment vitiliginous areas, methoxsalen is given in a dose of up to 600 micrograms/kg by mouth 2 to 4 hours before measured periods of exposure to UVA twice a week, at least 48 hours apart.
Methoxsalen may also be applied topically to repigment small, well-defined vitiliginous lesions. Preparations containing up to 1% have been used but dilution to 0.1 or 0.01% may be necessary to avoid adverse cutaneous effects. The surrounding skin should be protected by an opaque sunscreen. Some suggest that the treated area should be exposed to UVA immediately after application while others recommend waiting up to 2 hours. After exposure the lesions should be washed and protected from light; protection may be necessary for up to 48 hours or longer. Treatment is repeated usually once weekly. Significant repigmentation may not appear until after 6 to 9 months of treatment.
For the treatment of psoriasis a similar schedule is used to that outlined above for vitiligo. A dose of up to about 600 micrograms/kg by mouth 2 hours before UVA is usually given twice a week although increased frequencies, but with at least 48-hour intervals between doses, have been suggested. If there is no response or only minimal response after the fifteenth PUVA treatment some suggest that the dosage may be increased, once only, by 10 mg for the remainder of the course of treatment.
Methoxsalen may also be used topically with UVA exposure for the treatment of psoriasis. For direct application to affected areas of skin a preparation containing approximately 0.15% (or diluted to 0.015% if necessary to avoid adverse cutaneous effects) is applied 15 minutes before UVA exposure. Alternatively the patient may take a whole-body bath for 15 minutes in a methoxsalen solution, followed immediately by UVA exposure. UK guidelines (see also ) suggest a typical concentration of methoxsalen 2.6 mg/litre for such solutions although higher concentrations (up to about 3.7 mg/litre) have been used. Hand and foot soaks may be used to treat only those affected areas; a solution containing methoxsalen 3 mg/litre may be used with the affected areas immersed for 15 minutes followed by a delay of 30 minutes before UVA exposure. Baths or soaks are generally given twice a week.
Psoralen itself has also been used.
Administration.
The dose of methoxsalen is usually calculated on the basis of body-weight. This method of dose calculation produces a considerable difference between the doses received by heavy and light patients. A study in 41 patients with psoriasis1 suggested that using methoxsalen 25 mg/m2 gave more consistent plasma concentrations and may reduce the potential for burning in heavy patients and prevent underdosing in light patients undergoing PUVA therapy.
1. Sakuntabhai A, et al. Calculation of 8-methoxypsoralen dose according to body surface area in PUVA treatment. Br J Dermatol 1995; 133: 919–23. PubMed
PUVA.
PUVA combines psoralens with ultraviolet A irradiation. The psoralens may be given directly to the patient, either orally or topically, and the patient is then exposed to UVA. In extracorporeal PUVA (extracorporeal photochemotherapy; photopheresis), an oral dose of psoralen is administered, after which the patient's leucocytes are isolated, exposed to UVA extracorporeally, and then reinfused. In another method of photopheresis that is under investigation, methoxsalen is added directly to leucocytes that have already been removed from the patient. The mixture is then treated with UVA extracorporeally after which it is returned to the patient; the total dose of methoxsalen used by this method is lower than that used orally. PUVA has been used in a wide range of disorders including skin disorders (), mycosis fungoides (), and organ and tissue transplant rejection ().
Mycosis fungoides.
PUVA therapy is used in the treatment of the manifestations of cutaneous mycosis fungoides and Sèzary syndrome, two forms of cutaneous T-cell lymphoma (see ). Extracorporeal PUVA therapy (photopheresis; see ) has also been used,1-4 particularly for disease with erythrodermic features.
1. Duvic M, et al. Photopheresis therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol 1996; 35: 573–9. PubMed
2. Zic JA, et al. Long-term follow-up of patients with cutaneous T-cell lymphoma treated with extracorporeal photochemotherapy. J Am Acad Dermatol 1996; 35: 935–45. PubMed
3. Zic JA, et al. The North American experience with photopheresis. Ther Apher 1999; 3: 50–62. PubMed
4. Rubegni P, et al. Extracorporeal photochemotherapy in long-term treatment of early stage cutaneous T-cell lymphoma. Br J Dermatol 2000; 143: 894–6. PubMed
Organ and tissue transplantation.
PUVA and extracorporeal PUVA therapy (photopheresis; see ) have been tried in patients with graft-versus-host disease1-3 and to prevent rejection in cardiac4 and other solid organ transplantation.2
1. Konstantinow A, et al. Chronic graft-versus-host disease: successful treatment with extracorporeal photochemotherapy: a follow-up. Br J Dermatol 1996; 135: 1007–8. PubMed
2. Zic JA, et al. The North American experience with photopheresis. Ther Apher 1999; 3: 50–62. PubMed
3. Kunz M, et al. Treatment of severe erythrodermic acute graft-versus-host disease with photochemotherapy. Br J Dermatol 2001; 144: 901–2. PubMed
4. Barr ML, et al. Photopheresis for the prevention of rejection in cardiac transplantation. N Engl J Med 1998; 339: 1744–51. PubMed
Skin disorders.
PUVA has been used in a wide range of skin disorders and guidelines have been published by the British Photodermatology Group,1,2 which are summarised as follows:
Indications for PUVA in chronic plaque psoriasis include severe extensive psoriasis unresponsive to conventional topical therapies, relapse within 3 to 6 months of successful topical treatment, or patient refusal of topical treatment if UVB phototherapy has failed (see for a discussion of the various treatments of psoriasis). Initial UVA exposure should preferably be determined on the basis of prior measurement of the minimal phototoxic dose rather than on the skin type. Increases in UVA irradiation are then calculated as a percentage of previous doses.
Methoxsalen in a dose of 600 micrograms/kg by mouth given 2 hours before UVA exposure is the widely accepted standard regimen. Alternatively, 5-methoxypsoralen 1.2 mg/kg, again 2 hours before UVA exposure, can be given and appears to be almost free of the adverse reactions such as nausea, pruritus, and erythema induced by methoxsalen. However, until the clinical efficacy of 5-methoxypsoralen has been clearly demonstrated, methoxsalen should remain the psoralen of choice for most clinical situations.
Alternatives to oral PUVA are baths or soaks using methoxsalen or trioxysalen. For whole-body bathing a concentration of methoxsalen 2.6 mg/litre is typically utilised with the patient bathing for 15 minutes followed by immediate exposure to UVA. For hand and foot soaks a concentration of methoxsalen 3 mg/litre is used with the affected area immersed for 15 minutes followed by a delay of 30 minutes before UVA exposure. For trioxysalen a concentration of about 330 micrograms/litre is used for a 15-minute whole-body bath or hand and foot soak followed by immediate UVA exposure for whole-body therapy, or a 30 minute delay before hand and foot UVA exposure. Whole-body baths or hand and foot soaks are given twice each week.
Methoxsalen may also be applied topically to the affected areas. A concentration of about 0.15% (or 0.015% if erythema occurs) is used in an emulsion, or 0.005% in an aqueous gel, and applied 15 minutes before UVA exposure. PUVA treatment should be discontinued as soon as clearance is achieved and maintenance PUVA after routine clearance should be avoided.
A combination of PUVA with acitretin (300 to 700 micrograms/kg by mouth) or etretinate (0.5 to 1 mg/kg by mouth) may be considered in patients who have reached 50 treatment sessions or relapsed within 6 months of PUVA. PUVA and methotrexate are effective for severe psoriasis but should be reserved for such cases because of the possible increased risk of skin cancer
Oral PUVA twice weekly with methoxsalen 600 micrograms/kg or 5-methoxypsoralen 1.2 mg/kg has been effective in many patients with vitiligo (see Pigmentation Disorders, ). If patches are large and well demarcated topical application of methoxsalen 0.15% may be preferable
In mycosis fungoides PUVA is an effective symptomatic treatment for early disease and a useful adjunct for late-stage disease but optimal regimens have not been established (see )
PUVA is effective for atopic eczema () but clearance is less certain than for psoriasis, twice the number of treatments may be needed, and relapse is more frequent. It should therefore be reserved for severe disease unresponsive to conventional treatments. Optimal regimens have not been established
In polymorphic light eruptions PUVA is effective in up to 90% of patients but is only indicated in those who are frequently or severely affected despite the regular use of high-protection broad-spectrum sunscreens. Several arbitrary regimens are in use
Variable results have also been reported in a variety of other disorders but data has been insufficient to establish precise guidelines. Such disorders include actinic prurigo, alopecia areata, aquagenic pruritus, chronic actinic dermatitis, granuloma annulare, lichen planus, nodular prurigo, pityriasis lichenoides, localised scleroderma, solar urticaria, and urticaria pigmentosa. In most cases relapse occurs in the absence of maintenance therapy and PUVA should usually only be tried as a last resort.
Extracorporeal PUVA has been tried in patients with severe epidermolysis bullosa acquisita,3,4 and scleroderma.5
1. British Photodermatology Group. British Photodermatology Group guidelines for PUVA. Br J Dermatol 1994; 130: 246–55. PubMed
2. Halpern SM, et al. Guidelines for topical PUVA: a report of a workshop of the British Photodermatology Group. Br J Dermatol 2000; 142: 22–31. PubMed Also available at: online (accessed 22/12/05)
3. Miller JL, et al. Remission of severe epidermolysis bullosa acquisita induced by extracorporeal photochemotherapy. Br J Dermatol 1995; 133: 467–71. PubMed
4. Gordon KB, et al. Treatment of refractory epidermolysis bullosa acquisita with extracorporeal photochemotherapy. Br J Dermatol 1997; 136: 415–20. PubMed
5. Zic JA, et al. The North American experience with photopheresis. Ther Apher 1999; 3: 50–62. PubMed
Preparations
Single-ingredient Preparations
The symbol ¤ denotes a preparation which is discontinued or no longer actively marketed.
Australia: Oxsoralen; Austria: Oxsoralen; Belgium: Mopsoralen; Oxsoralon¤; Brazil: Oxsoralen; Canada: Oxsoralen; Ultramop; Chile: Oxsoralen; Czech Republic: Oxsoralen; Denmark: Geroxalen; France: Meladinine; Germany: Meladinine; Greece: Melaoline; Hong Kong: Oxsoralen; Hungary: Geroxalen; India: Macsoralen; Manaderm; Melanocyl; Ireland: Deltasoralen; Israel: Oxsoralen¤; Italy: Oxsoralen¤; Japan: Oxsoralen; Malaysia: Meladinine¤; Oxsoralen; Mexico: Dermox; Meladinina; Oxsoralen; The Netherlands: Geroxalen; Meladinine; Oxsoralen; Norway: Geroxalen; New Zealand: Oxsoralen; South Africa: Oxsoralen; Singapore: Oxsoralen; Spain: Novo Melanidina¤; Oxsoralen; Switzerland: Meladinine; Thailand: Meladinine¤; United Kingdom: Puvasoralen; United States: Oxsoralen; Uvadex;
Multi-ingredient Preparations
The symbol ¤ denotes a preparation which is discontinued or no longer actively marketed.
India: Melanocyl;
Pharmacopoeial Preparations
USP 29: Methoxsalen Capsules; Methoxsalen Topical Solution;
imi cer mii de scuze...dar n.am timp sa.l traduc si consider ca e super important!
Anca si Alexandra (10 nov.2003)
poze:
http://community.webshots.com/user/alexandra_ioana

 getak spune:
getak spune:
Buna tuturor! Deoarece nu prea ma descurc cu engleza si nu am inteles prea multe din articol, am cautat in spaniola despre methoxsalen si am gasit ceva important, cred eu.Methoxsalen(meth- BUEY-uno-len)se utilizeaza impreuna cu lumina ultraviolet(UVA)(gasita in lumina soarelui sau lampi speciale) pentru a trata vitiligo sau psoriazis. In primul rand tratamentul se face doar la indicatia si sub supravegherea medicului, daca nu e folosit corect tratamentul poate cauza arsuri, cancer de piele, cataracta, imbatranirea prematura a pielii. Efectele se observa dupa 6-8 saptamani si in nici un caz nu trebuie crescuta doza si nici timpul de expunere la soare iar in timpul tratamentului se evita consumarean unor alimente ca:patrunjel, morcovi, mustar, spanac.Pentru vitiligo sunt indicate 20 mg pe zi cu 2-4 ore inainte de expunere la soare si se ia de 2 sau 3 ori pe saptamana. Trebuie regulat sa mergi la doctor sa urmareasca progresele precum si eventualele efecte nedorite totodata este necesar control oculuist. Inainte de fiecare tratament nu trebuie sa te expui la soare cel putin 24ore. Buzele trebuie protejate cu creion de buze cu SFP15. Trebuie folosit un produs de proterctie a pielii cu SFP de cel putin 15 dar numai pe zonele fara pete. Dupa fiecare tratament se evita soarele cel putin 8 ore iar deoarece pielea continua sa ramana sensibila la soare multe ore dupa tratament trebuie sa se aiba grija cu expunerea la soare cel putin 48 ore dupa tratament.24 ore dupa fiecare doza de methoxsalen, ochii trebuie protejati in timpul zilei cu ochelari de soare speciali care blocheaza sau absorb razele ultraviolete (nu ochelari de soare obisnuiti) pentru a preveni cataracta. Ca efecte secundare pot aparea: usturimi ale pielii,greata,dureri de cap, depresie, nervozitate si somnolenta. Acestea sunt indicatiile care le-am gasit sper sa va ajute, mie cel putin mi-au inlaturat unele nedumeriri in legatura cu medicamentul si o sa incerc sa le urmez.
trebuie sa se aiba grija cu expunerea la soare cel putin 48 ore dupa tratament.24 ore dupa fiecare doza de methoxsalen, ochii trebuie protejati in timpul zilei cu ochelari de soare speciali care blocheaza sau absorb razele ultraviolete (nu ochelari de soare obisnuiti) pentru a preveni cataracta. Ca efecte secundare pot aparea: usturimi ale pielii,greata,dureri de cap, depresie, nervozitate si somnolenta. Acestea sunt indicatiile care le-am gasit sper sa va ajute, mie cel putin mi-au inlaturat unele nedumeriri in legatura cu medicamentul si o sa incerc sa le urmez.

 gabi30 spune:
gabi30 spune:
Buna.
Tocmai m-am dat cu autobronzant pe zonele depigmentate.Sunt tare curioasa ce o sa iasa.Nu am reusit sa dau numai pe pete.Am nimerit si pe langa.Oricum la cat sunt de bronzata pe zonele sanatoase nu cred ca mai conteaza.Diseara mai dau o tura si sper sa arat cat de cat respectabil in weekend.
In rest continui tratamentul.Rezultatele tot apar.Intra pigmentul destul de bine.Nu pe toate petele la fel dar asta e.Chiar si in palma!Nici nu mi-am dat seama ca si acolo sunt probleme.
La voi cum merge?
Gabi

 gabi30 spune:
gabi30 spune:
Buna.
Nu a prins autobronzantul pe pete!
M-am simtit putin ciudat dar nu s-a uitat nimeni la mine foarte curios.Mai mult copii decat adultii.
Saptamana asta nu am stat inca la soare.Sper sa pot sta maine vreo 2 ore dimineata.
Cum merge tratamentul?
Gabi

 arinna spune:
arinna spune:
Gabi, felicitari pentru rezultate ! Eu nu fac tratamenul cu melaoline dar urmaresc ce scrieti, ma bucur sa aud vesti bune...Cum mai e cu soarele, nu e greu de stat? cat mai stai pe zi?Eu nu stiu daca as rezista pe caldura asta...:(
felicitari pentru rezultate ! Eu nu fac tratamenul cu melaoline dar urmaresc ce scrieti, ma bucur sa aud vesti bune...Cum mai e cu soarele, nu e greu de stat? cat mai stai pe zi?Eu nu stiu daca as rezista pe caldura asta...:(
........................
Knowledge is power!

 anca tanasica spune:
anca tanasica spune:
sotul meu a inceput sa se duca la solar seara din doua in doua zile...da nu.i decat o sapt.de cand se duce...s.a inrosit mult mai tare pe toate petele...pe frunte nu a intrat inca pigmentul dar petele is asa de rosii ca nici nu se mai vad...e super bronzat si asteptam pigmentul...mergem si la mare o sapt si ma gandesc ca si stat pe plaja mult va ajuta...
Anca si Alexandra (10 nov.2003)
poze:
http://community.webshots.com/user/alexandra_ioana

 gabi30 spune:
gabi30 spune:
buna
eu nu am reusit sa stau la soare deloc saptamana asta
nu a fost soare
la solar nu ma lasa sotul sa merg , zice ca nu e sanatos deloc si oricum pe el nu il deranjeaza petele deloc , din partea lui pot sa nu mai fac nici un tratament...
cam cate minute se sta la solar?
sper ca in weekend sa prin macar 2 zile cu soare
nu stiu daca e de la pastile dar mi-a fost cam rau de la ele de vreo 2 ori...am si vomat...
gabi

 anca tanasica spune:
anca tanasica spune:
quote:
Originally posted by gabi30
buna
eu nu am reusit sa stau la soare deloc saptamana asta
nu a fost soare
la solar nu ma lasa sotul sa merg , zice ca nu e sanatos deloc si oricum pe el nu il deranjeaza petele deloc , din partea lui pot sa nu mai fac nici un tratament...
cam cate minute se sta la solar?
sper ca in weekend sa prin macar 2 zile cu soare
nu stiu daca e de la pastile dar mi-a fost cam rau de la ele de vreo 2 ori...am si vomat...
gabi
sotul meu sta 5 min pe o parte si 5 pe partea cealalta la solar...nu.l las eu mai mult...
...rau nu i.a fost in aceasta perioada de cand ia pastilele...dar ii voi cumpara un liv52 pentru ficat...
Anca si Alexandra (10 nov.2003)
poze:
http://community.webshots.com/user/alexandra_ioana

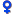
 EllaB spune:
EllaB spune:
Salutari la toata lumea!
eu am 27 ani si am vitiligo de o gramada de timp, cam de cand cu cernobalu', asa ziceau si doctorii la inceput ca de acolo mi se trage. Eu zic totusi ca mi-a aparut pe fond nervos, deoarece am fost un copil deosebit de sensibil (acuma m-am mai calit  ), sufeream si plangeam din orice.Am incercat si o gramada de tratamente, ultimul pe care il fac acum e cu daivonex, de vreo 3 luni, dar inca nu am observat nimic deosebit. M-am chinuit de-a lungul timpului si cu tot felul de tincturi, am baut ulei de masline in fiecare dimineata cate un paharel cateva luni, (ca asta mi-a zis cineva ca ar fi medicamentul minune), dar singurul tratament care a avut ceva efect (probabil daca ma tineam ar fi avut si mai mult) a fost chiar primul facut la D-na dr. Cristodulo cu oxsoralen. Atunci mi-au disparut 2 pete de pe fata. Daca as fi locuit in bucuresti, probabil ca ar fi fost cu totul altceva, dar cu deplasarea a fost destul de greu.
), sufeream si plangeam din orice.Am incercat si o gramada de tratamente, ultimul pe care il fac acum e cu daivonex, de vreo 3 luni, dar inca nu am observat nimic deosebit. M-am chinuit de-a lungul timpului si cu tot felul de tincturi, am baut ulei de masline in fiecare dimineata cate un paharel cateva luni, (ca asta mi-a zis cineva ca ar fi medicamentul minune), dar singurul tratament care a avut ceva efect (probabil daca ma tineam ar fi avut si mai mult) a fost chiar primul facut la D-na dr. Cristodulo cu oxsoralen. Atunci mi-au disparut 2 pete de pe fata. Daca as fi locuit in bucuresti, probabil ca ar fi fost cu totul altceva, dar cu deplasarea a fost destul de greu.
In general eu sunt destul de impacata cu petutzele mele si daca as stii ca raman asa cum sunt, probabil ca nu m-as mai chinui cu tratamentele. Bine, am avut perioada mea de complexe, dar cu cat am inceput sa-mi dezvolt talentele in le fardatului, m-am mai linistit.
Cele mai mari care le am sunt pe pleoape si ce ma deranjeaza e ca la unul din ochi a inceput sa coboara si sub pleoape. Foarte lent, e adevarat, dar parca totusi se misca.
Am citit aici pe forum despre novitil si ma gandesc sa trec la actiune, dar mai astept putin.
De ceva timp eu si cu sotul meu ne dorim ffff mult un bebe. Dar eu tot aman de frica (pe langa cea de nastere :)) sa nu transmit si copilului boala, cu toate ca eu nu am avut pe nimeni in familie, sau cel putin nimeni nu-si aduce aminte de asa ceva. Asa ca le rog pe
toate mamicile ca mine de pe acest forum sa-mi spuna cum sunt bebelasii lor. Si mai vreau sa stiu cum s-au modificat petele mamicilor in timpul sarcinii si daca ati facut vreun tratament extern (unguent) in acest timp. Va rog f mult sa ma ajutati cu experientele voastre ca asa poate mai prind curaj.
Va pup pe toate si va doresc sa se faca bebeii mari si cel mai
important, sanatosi.
